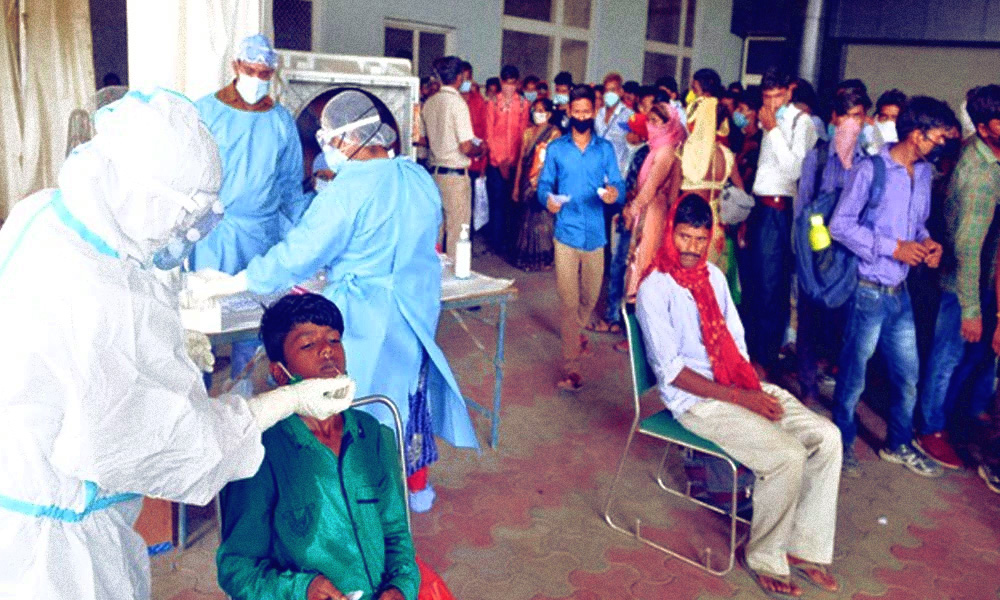
Image Credits: ANI
Drug Regulator Panel Recommends Phase I Trial For Bharat Biotech's Intranasal COVID-19 Vaccine
Writer: Rishab Shaju
A passionate, confident, and energetic student, I am a workaholic with an interest in the field of Broadcast Journalism. I always make sure to meet my deadlines and can work well under pressure. Other than journalism, I am also interested in the field of Psychology and Literature. Timeliness and honesty are the two most important factors that define me. If not journalism, I would want to be a professor or a social worker.
India, 23 Jan 2021 7:20 AM GMT
Editor : Prateek Gautam |
A free soul who believes that journalism, apart from politics, should stand for social cause and the environment.
Creatives : Abhishek M
" An engineer by profession, Abhishek is the creative producer of the team, graphic designing is his passion and travelling his get away. In more ways than one, he makes the content visually appealing."
NITI Aayog member, Dr VK Paul, said, "If this vaccine works, it can be a game-changer because it is so easy to use and we look forward to this development."
The Central Drugs Standard Control Organisation (CDSCO), on Tuesday, January 19, recommended granting permission for conducting the Phase I clinical trial of an intranasal vaccine against COVID-19, which has been developed by Bharat Biotech.
The NITI Aayog member, Dr VK Paul, said, "If this vaccine works, it can be a game-changer because it is so easy to use and we look forward to this development." According to the news agency Press Trust of India, the Bharat Biotech had applied to the Drugs Control General of India, seeking permission for conducting the Phase I and Phase II clinical trials of the intranasal vaccine. The committee then recommended granting permission for the trials of the vaccine.
"Based on the safety and immunogenicity data of Phase I clinical trial, the company would be given permission for conducting Phase II clinical trial," said an official.
As per reports, the vaccine maker said, "BBV154 (intranasal COVID-19 vaccine) pre-clinical testing has been completed for toxicology, immunogenicity, and challenge studies. These studies have been conducted in the USA and India. Phase I human clinical trials will commence during February-March 2021."
Earlier information revealed by Dr Paul said that this could be an exciting development because potentially, by using an intranasal vaccine, the antigen could be delivered with much more safety, and hence an immunological response would also happen rapidly.
The Chairman of Bharat Biotech, Dr Krishna Ella, had earlier said that the company had been focussing on the intranasal vaccine as existing vaccines require a two-dose intramuscular injection and a country like India would need close to 2.6 billion syringes and needles, which may then add to pollution as well. "One drop of vaccine in each of the nostrils is sufficient," he had said.
However, Dr Ella also said that the vaccine may not be simple to administer, but, it will reduce the use of medical consumables such as needles, syringes, etc. significantly impacting the overall cost of the vaccination drive in the country.
Also Read: Karnataka: Resident Doctors Demand Choice Of COVID-19 Vaccine Before Vaccination
 All section
All section